
Menu

Point of Care (clinic and home) identification of OAD conditions and worsening trends through a comprehensive new platform that seamlessly captures airway oscillometry, inhaler adherence + technique, symptoms, and FEV1.
PulmoScan – first oscillometry device FDA-cleared for home use

Data to be presented at the ATS session: Poster #P590: Identifying Obstructive Airway Disease in Adults Via a Neural Network-based Diagnostic Classifier Employing Lung Oscillometry
A75 – REVISITING CURRENT METHODS IN PULMONARY FUNCTION TESTING
– May 19 11:30 AM – 1:15 PM
– Poster Board # P590, Area E (Hall A-B2, Ground Level).


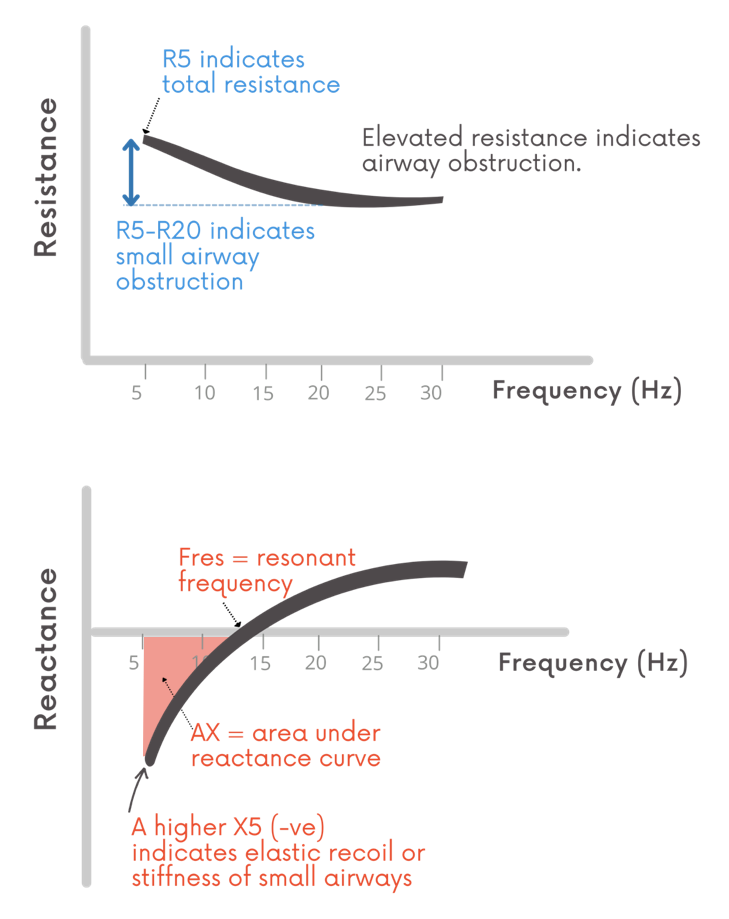
PulmoScan can assess obstruction via two markers measured for peripheral and central airways:






See a live demo of the PFT and home use devices and implementation workflow for your practice/research. Fill the form out below and we will get in touch with you at your preferred time. Don’t worry, we don’t spam and you can unsubscribe at any time.